世界上第一個人類生物材料(Humabiologics)
目前使用的異源性基材跟人類來源基材仍存在差異?
Humabiologics讓您的實驗條件更貼近人類組織器官
- 人類來源,更貼近臨床
- cGMP下生產
- 價格實惠、供應穩定和高質量
- 多項發表及研究使用
- HIV-1 、 HIV-2、乙型肝炎、丙型肝炎以及其他傳染源呈陰性
- 經美國組織庫協會 (AATB) 認證皆符合FDA 21CFR1271法規和標準
世界上第一個源自人類的生物材料
在再生醫學領域,使用了多種生物材料,包括天然聚合物、合成聚合物或兩者的混合物。合成聚合物具有優異的機械性能,例如高強度,而天然聚合物具有優異的生物性能,例如生物相容性,通常用於製造人體組織。天然聚合物,如膠原蛋白、明膠、透明質酸、海藻酸和殼聚醣,多年來因其在再生醫學中的應用而被廣泛研究。在保持其優異的生物特性的同時,這些天然聚合物還可以調整其機械特性,使其更適合再生醫學應用。膠原蛋白及其衍生物明膠一直受到研究人員的特別關注,因為大多數人體組織,包括皮膚、骨骼、肌肉和器官,都是由膠原蛋白製成的。
近年來,該領域以令人難以置信的速度發展,人們越來越認識到組織製造需要比單一蛋白質更複雜的材料。這導致許多研究人員使用細胞外基質 (ECM),它由多種蛋白質和生長因子組成,例如彈性蛋白、糖胺聚醣和非 I 型膠原蛋白。
然而,許多可用於開發人類治療的生物材料都來自動物,如大鼠、牛和豬。儘管這些生物材料與源自人類的生物材料有許多相似之處,但也存在差異,在人類治療的發展中,而這些差異對細胞反應和組織重塑有重大影響。
為了讓研究人員在實驗室中製造微型器官或利用生物列印製造人體組織,迫切需要開發在生理和臨床上更適合人類的生物材料。
人源產品出現的契機
十多年前,Humabiologics執行長Mohammad Albanna 博士正在為嚴重燒傷患者進行生物列印人體皮膚的研究,在需要人體生物材料的狀況下,找不到符合需求的產品。而目前創建出來可移植的人類皮膚,皆是利用次優的生物材料,即異源的基材,例如鼠尾的膠原蛋白。
而組織銀行利用捐贈的人體組織和器官來幫助盡可能多的人,但移植組織有的嚴格的標準,會因組織不匹配等狀況,而沒有問題的組織不用於移植而被丟棄。
接下來的十年裡,再生醫學領域有許多進步,但人源生物材料產品的需求、高品質、持續可用且負擔得起的人源生物材料的短缺仍然沒有解決的跡象。
Humabiologics 充當捐贈者組織和研究人員之間的橋樑
Huumabiologics從捐贈但不能用於移植的器官和組織中提取人體生物材料,並將其提供給研究人員。銷售世界上第一個從人類皮膚中提取的用於再生醫學的人類明膠和凍乾膠原蛋白。
產品所使用的組織嚴格來自器官採購組織 (OPO)、美國組織庫協會 (AATB) 認證、美國食品藥物管理局 (FDA) 註冊、符合 FDA 21 CFR 1271 的組織庫。

- 幫助各種人源細胞生長的培養基
- 用於藥物發現研究和篩選的類器官開發
- 作為列印人體組織和器官的材料
多篇paper使用
Humabiologics 人類來源產品 publications
| 系列 | Tile | 作者 | 年分 | 期刊 | 連結 |
|---|---|---|---|---|---|
| Human Collagen | 3D bioprinting of an implantable xeno-free vascularized human skin graft | Baltazar, T., Jiang, B., Moncayo, A., et al. | 2022 | Bioeng Transl Med. | Read More |
| Native human collagen type I provides a viable physiologically relevant alternative to xenogeneic sources for tissue engineering applications: A comparative in vitro and in vivo study. | Baltazar, T., Kajave, N.S., Rodriguez, M., et al. | 2022 | J Biomed Mater Res. | Read More | |
| In vitro characterization of xeno-free clinically relevant human collagen and its applicability in cell-laden 3D bioprinting. | Schmitt, T., Kajave, N., Cai H.H., Gu L., Albanna M., Kishore V. | 2021 | Journal of Biomaterials Applications. | Read More | |
| Species-Based Differences in Mechanical Properties, Cytocompatibility, and Printability of Methacrylated Collagen Hydrogels | Ali, S., Patrawalla, N.Y., Kajave, N.S., et al. | 2022 | Biomacromolecules | Read More | |
| Human Gelatin | Human gelatin-based composite hydrogels for osteochondral tissue engineering and their adaptation into bioinks for extrusion, inkjet, and digital light processing bioprinting | Bedell, M., et. al. | 2022 | Biofabrication | Read More |
| Leveraging multi-material bioprinting to examine the effect of architecture on mesenchymal stem cell-laden constructs’ tissue integration within an ex vivo osteochondral explant model | Bedell, M., et al | 2022 | - | Read More | |
| Dantrolene inhibits lysophosphatidylcholineinduced valve interstitial cell calcific nodule formation via blockade of the ryanodine receptor | Sylvester, C., et al. | 2023 | Front Cardiovasc Med | Read More | |
| Human ECM | Chemical Modification of Human Decellularized Extracellular Matrix for Incorporation into Phototunable Hybrid-Hydrogel Models of Tissue Fibrosis | Hewawasam R., et al., | 2023 | ACS Applied Materials & Interfaces | Read More |
| Tissue ECM | Collagen and Beyond: A Comprehensive Comparison of Human ECM Properties Derived from Various Tissue Sources for Regenerative Medicine Applications | Patrawalla, N.Y., et.al. | 2023 | Funct. Biomater | Read More |

In this publication, a successful method was developed for creating human skin substitutes using xeno-free isolation, expansion, and cryopreservation of human endothelial cells, fibroblasts, pericytes, and keratinocytes. The skin substitutes were printed using a xeno-free bioink containing human collagen type I and fibronectin and a biocompatible polyglycolic acid mesh. When implanted in immunodeficient mice, the skin substitutes developed a mature stratified epidermis with rete ridgelike structures and human EC-lined perfused microvessels. This demonstrates the feasibility of a xeno-free approach to complex tissue engineering.


Researchers compare the physico-chemical properties and suitability for tissue engineering human collagen type I-based hydrogels versus animal-derived collagen type I-based hydrogels. The study found that species and tissue-specific variations of collagen sources significantly impact the physical, chemical, and biological properties of collagen hydrogels, and that not all sources of human collagen behave similarly. The results suggest that commercially available human collagen can be used in place of xenogeneic sources to create functional scaffolds, but factors such as gelation kinetics, swelling ratio, collagen fiber morphology, compressive modulus, stability, and metabolic activity of cells must be considered in the development of 3D tissues for drug screening and regenerative medicine applications.


The study aimed to evaluate the potential use of xeno-free human skin-derived collagen as a bioink for 3D bioprinting in tissue engineering. The researchers compared the physical and chemical properties of human collagen hydrogels with those derived from bovine collagen. Results showed that the polymerization rate and compressive modulus of human collagen increased significantly with an increase in concentration. Raman spectroscopy also revealed that human collagen hydrogels had a denser and more organized fibrillar structure. Using 6mg/mL human collagen as a bioink led to the creation of 3D printed constructs with high shape fidelity and cell viability, while xenogenic collagen failed to produce stable 3D printed constructs. The findings suggest that human-derived collagen may be a viable alternative to xenogenic sources for 3D bioprinting of clinically relevant scaffolds in tissue engineering applications.


The article discusses the impact of variations in amino acid composition and collagen isolation method on the degree of methacrylation (MD) and subsequent physical properties of methacrylated collagen (CMA) hydrogels, as well as cell response. The authors compared the effects of three collagen species (bovine, human, and rat), two collagen extraction methods (pepsin digestion and acid extraction), and two photoinitiators on the physical properties of CMA hydrogels, printability, and mesenchymal stem cell (MSC) response. They found that human collagen showed the highest MD and yielded constructs with superior print fidelity. Lithium phenyl- 2,4,6-trimethylbenzoylphosphinate (LAP) was more cytocompatible than Irgacure- 2959 (I-2959), and the compressive modulus and cell viability of rat CMA were significantly higher than bovine CMA. The results suggest that careful selection of collagen source and cross-linking conditions is essential for designing biomimetic CMA hydrogels for tissue engineering applications.


The article discusses the application of novel hydrogel systems in osteochondral tissue engineering and the challenges of adapting these systems into 3D-printable bioinks. Results showed the relative integrals from three peaks present in the human gelMA spectra indicated a higher total degree of methacrylation and methacryloylation compared to the two peaks present in the methacryl region for the porcine gelMA. Confirmation of the higher degree of modification in the human gelMA was observed through a significant reduction in the lysine peak, which was highest in the unmodified porcine gelatin control group. Human gelMA samples at both β-TCP levels had significantly higher crosslinked storage moduli compared to porcine gelMA samples, indicating that the human gelMA was more gel-like than the porcine gelMA, which was also reflected in the qualitative handling of the gels using forceps. Changes in lyophilized mass revealed a significant decrease over time in the porcine gelMA samples during the first week of PBS incubation, dropping to 14.1 ± 11.0% remaining mass on day 8, while the human gelMA samples' mass decreased to a much lower magnitude, dropping to 79.8 ± 3.14% remaining mass on day 8. The human gelMA groups achieved 95% of their max storage moduli values more quickly than the porcine gelMA groups, taking 92 s compared to 216 s. The results indicate the potential of these novel composite hydrogel formulations using human gelMA as the base polymer for promoting osteochondral phenotypes and enabling the bioprinting of viable, cell-encapsulating constructs using multiple 3D printing methods.

The feasibility of integrating hMSCs into fresh bioink compositions was assessed through the utilization of luminescent ATP assays, CellTox Green, and Live/Dead staining to evaluate viability and growth. These techniques revealed significant distinctions when compared to the 2-hour timepoint in identical cohorts. The highest DNA content was observed at day 0 for human gelMA samples without β-TCP. After 14 days, human GelMA demonstrated higher Ca2+-bound Alizarin Red S stain than porcine GelMA, and higher DNA content was observed at days 0 and 14. Additional tests were conducted to compare biocompatibility outcomes linked to the gelMA backbone of the bioink. Results indicate a remarkable difference in comparison to day 0 within the same group and between gelMA samples with the same β-TCP level at a given timepoint. Due to the double-functionalization of the human gelMA, its ability to maintain crosslinked gel integrity over extended incubation periods, and its favorable outcomes in terms of viability and calcium deposition, it was selected as the primary bioink backbone polymer for all subsequent differentiation and printability investigations.
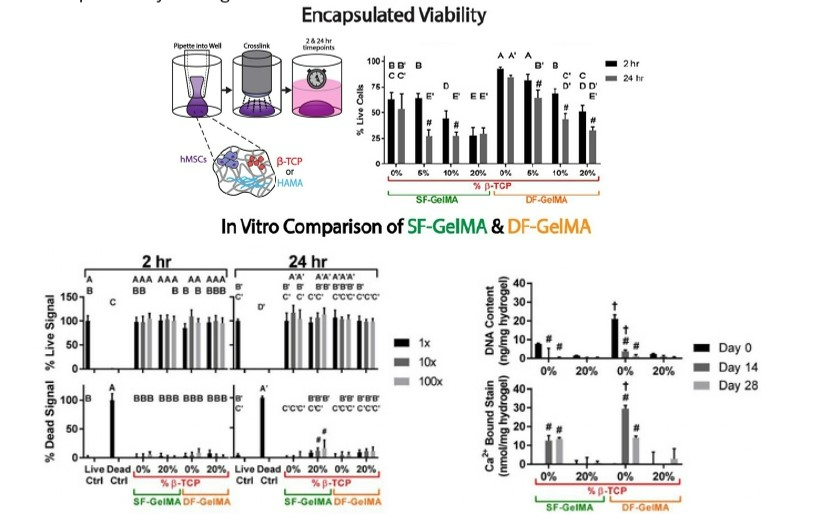
A study on differentiation utilized human GelMA as the primary polymer in the bioink. Findings demonstrated that the presence of 10% w/v β-TCP led to an increase in the level of matrix mineralization when utilized in mixed media conditions. To assess printability and the extent to which cell growth and proliferation were supported by each ink, hMSCs were enclosed and printed in the bioinks, cultivated in multi-well plates, and examined for ATP content along with nonprinted samples of each formulation for up to 7 days following printing. The bioinks with the highest observed printability were selected for the study.


Novel bioprinted constructs for osteochondral tissue engineering were fabricated to study the effect of multi-material architecture on encapsulated human mesenchymal stem cells’ tissue-specific matrix deposition and integration into an ex vivo porcine osteochondral explant model. Two extrusion fiber architecture groups with differing transition regions and degrees of bone- and cartilage-like bioink mixing were employed. Both inks were formulated with 10% w/v human gelMA, 0.5% w/v LAP, 1% w/v xanthan gum (XG), 2% w/v nano-fibrillated cellulose (NFC). Use of this three-week osteochondral model demonstrates that these novel bioink formulated with human gelMA, support the fabrication of cell-laden constructs that integrate into explanted tissue as capably as natural tissue and encapsulate osteochondral matrix-producing cells, and it also highlights the important role that spatial architecture plays in the engineering of multi-phasic tissue environments.


The only treatment for end-stage calcific aortic valve disease (CAVD) is invasive valve replacement, making the development of pharmaceutical treatments that can slow or reverse CAVD progression crucial. To investigate whether blocking calcium signaling in valvular interstitial cells (VIC) could mitigate calcification, the effects of dantrolene on Porcine aortic VICs (paVICs) were studied in an in vitro model. Encapsulating paVICs in human gelatin methacrylate showed that the 3-dimensional microenvironment had different effects on the cells when treated with lysophosphatidylcholine (LPC) and dantrolene. Findings suggest that ryanodine receptor isotope 3 (RyR3) is a viable therapeutic target for CAVD treatment, warranting further investigations into the effects of RyR3 inhibition on CAVD.


The persistent occurrence of tissue fibrosis is a severe health concern that is associated with high rates of morbidity and mortality. In this study, a process is presented for modifying human decellularized extracellular matrix (dECM) chemically and integrating it into a hybrid-hydrogel system that is dynamically tunable and contains a poly(ethylene glycol)-α methacrylate (PEGαMA) backbone. By incorporating intact, tissue-derived dECM, key biochemical properties of human tissue were recapitulated while taking advantage of dual-stage photopolymerization techniques to mimic the dynamic increase in microenvironmental stiffness observed during fibrotic progression in human organs. The incorporation of dECM altered key network properties of the hydrogels, including increasing the swelling ratio, average molecular weight between crosslinks, and mesh size, while decreasing the average shear modulus of the resulting hybrid-hydrogels. These findings advance our knowledge of the biomolecular networks that form within hybrid-hydrogels. This completely human phototunable hybrid-hydrogel system allows researchers to regulate and disentangle the biochemical modifications that occur during fibrotic pathogenesis from the resulting increases in stiffness, allowing for the study of the dynamic cell−matrix interactions that perpetuate fibrotic diseases.


The physiochemical and biological properties of ECM hydrogels derived from four different human tissues: skin, bone, fat, and birth were studied to determine if there are significant differences between tissue sources for the development of ECMbased biomimetic tissue constructs for regenerative medicine applications. Results from assessing the decellularization efficacy showed that >90% of DNA was removed confirming decellularization from all tissues. Next, a hydroxyproline quantification assay was performed to quantify the total collagen content in each ECM and the SDS-PAGE analysis of the tissue-specific ECM revealed distinct bands at various molecular weights. These results show the efficiency of the decellularization process and align with existing knowledge that different tissues possess diverse ECM compositions. Quantification of gelation kinetics of different human tissue ECM solutions showed compositional variations in ECM derived from various tissues which suggest tissue types impact the gelation kinetics of each hydrogel. This further indicates that the time required for the initiation of collagen fibrillogenesis can vary depending on the tissue source and the specific composition of the ECM.

Researchers studied the surface morphology of human tissue ECM hydrogels along with their cell viability and their metabolic activity in 2D and 3D culture systems. SEM images showed that the hydrogels fabricated from various human tissues had differences in surface microstructure. The highly dense collagen fibers observed with SEM for birth and skin ECM might allow for enhanced cell-matrix interactions and signaling, thereby promoting higher metabolic activity. Cell attachment results exhibited excellent cell viability on day 1 for all the tissue-specific coated plates in 2D culture and displayed well-spread morphology on day 7, maintaining their viability overtime. A similar phenomena was shown for the 3D cell culture system wherein the cell metabolic activity was comparable in all hydrogels on day 1 and increased significantly overtime. Overall, these findings indicate that multicomponent ECM matrix can better support cell response, as cells cultured in 2D and 3D pure collagen systems was lower than tissue-specific ECM.

Rheological characteristics of ECM inks were studied by evaluating the change in viscosity of the inks with varying shear rate. All inks exhibited shear thinning properties indicated by the decrease in viscosity with increase in shear rate confirming their applicability in extrusion-based 3D bioprinting. Printability of the tissue-specific ECM inks showed that comparable drop sizes were obtained for collagen and skin ECM. Bone ECM and fat ECM also exhibited similar drop sizes. Birth ECM showed the largest drop size with a significantly higher drop size than all other inks. These combined results revealed that skin ECM exhibits higher viscosity and superior printability compared to other ECMS, indicating that compositional differences in tissue-specific ECMs may influence the use of these materials as bioinks in 3D bioprinting applications.

